- Product Details
Keywords
- 98198-48-2
Quick Details
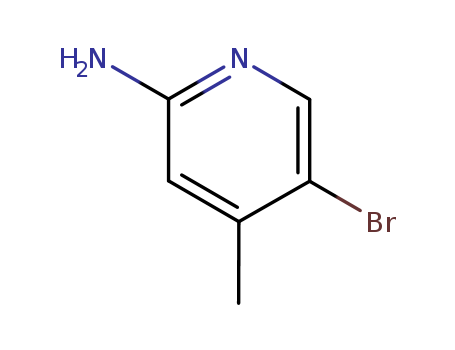
- ProName: 2-Amino-5-bromo-4-methylpyridine
- CasNo: 98198-48-2
- Molecular Formula: C6H7BrN2
- Appearance: Similar white to slight yellow solid
- Application: intermediates
- DeliveryTime: in stock
- PackAge: 25KG/NET Fiber Drum with double lined ...
- Port: beijing
- ProductionCapacity: 5 Metric Ton/Year
- Purity: 98%min(HPLC)
- Storage: Store in a cool, dry area. Keep contai...
- Transportation: NOT RESTRICTED
- LimitNum: 1 Kilogram
Superiority
1. Introduction of2-Amino-5-bromo-4-methylpyridine
2-Amino-5-bromo-4-methylpyridine (CAS NO.98198-48-2) is a similar white to slight yellow s
Details
1. Introduction of 2-Amino-5-bromo-4-methylpyridine
2-Amino-5-bromo-4-methylpyridine (CAS NO.98198-48-2) is a similar white to slight yellow solid and belongs to the Amino-pyridine series. We can supply its 98%min(HPLC) and produce it 5 metric ton per year. It is in stock now and can be sent from the beijing port. Whereas, please send the payment via the L/C or T/T.
Other specification:
Appearance :Similar white to slight yellow solid
CAS NO.: 98198-48-2
Assay :≥98.0%(HPLC)
Formula: C6H7BrN2
M.W.: 187.04
Feature: Similar white to slight yellow solid,m.p: 148-151℃
Packaging: 25KG/NET Fiber Drum with double lined plastic bag inside
2. Basic information of 2-Amino-5-bromo-4-methylpyridine
1) Properties:
(1)XLogP3-AA: 1.6; (2)H-Bond Donor: 1; (3)H-Bond Acceptor: 2; (4)Tautomer Count: 2; (5)Exact Mass: 185.97926; (6)MonoIsotopic Mass: 185.97926; (7)Topological Polar Surface Area: 38.9; (8)Heavy Atom Count: 9; (9)Complexity: 97.1; (10)Covalently-Bonded Unit Count: 1; (11)Feature 3D Donor Count: 1; (12)Feature 3D Cation Count: 1; (13)Feature 3D Hydrophobe Count: 1; (14)Feature 3D Ring Count: 1; (15)Effective Rotor Count: 0; (16)Conformer Sampling RMSD: 0.4; (17)CID Conformer Count: 1.
2) Descriptors of Structure
IUPAC Name: 5-bromo-4-methylpyridin-2-amine
InChI: InChI=1S/C6H7BrN2/c1-4-2-6(8)9-3-5(4)7/h2-3H,1H3,(H2,8,9)
InChIKey: JDNCMHOKWINDKI-UHFFFAOYSA-N
Canonical SMILES: CC1=CC(=NC=C1Br)N